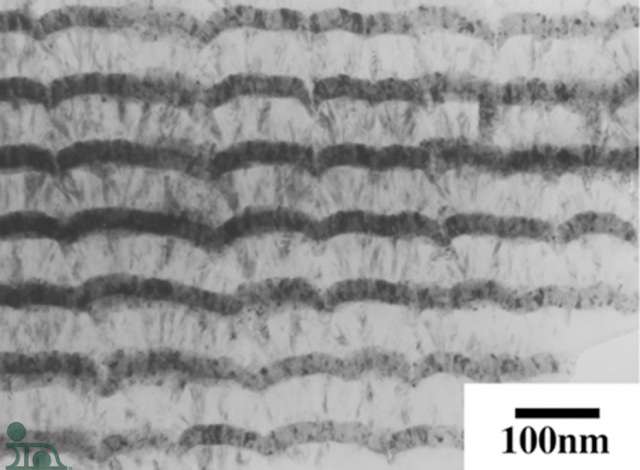
 One of the key points to understanding a NanoBond® application is understanding the reaction that takes place. When NanoFoil® is activated, the nickel and aluminum bilayers (picture on left) start a self-sustaining reaction that quickly consumes the material.
One of the key points to understanding a NanoBond® application is understanding the reaction that takes place. When NanoFoil® is activated, the nickel and aluminum bilayers (picture on left) start a self-sustaining reaction that quickly consumes the material.
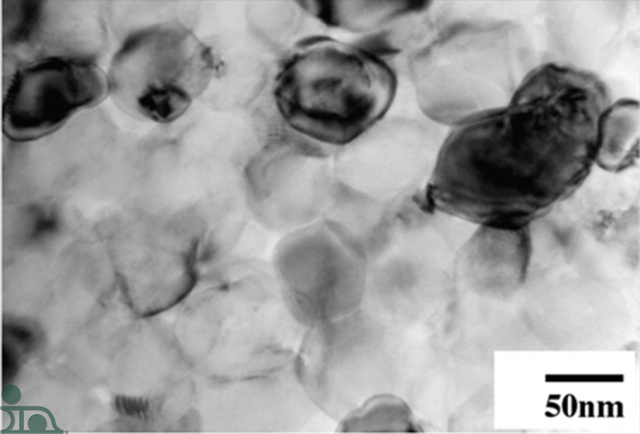
After the reaction takes place, the aluminum and nickel layers form a brittle intermetallic (picture on right). This bi-product, nickel aluminide, shrinks and cracks allowing solder to flow between and complete the interconnection.
Through a series of posts, we will discuss:
I know this doesn’t cover everything you’d ever want to know about the reaction of NanoFoil®, but that’s what we are here for! Let us know if you have a question.
*This post is part of the NanoBond® Process series


