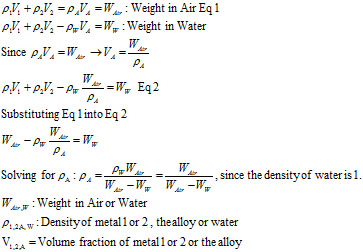Folks,
In the category of interesting requests, Ron, a gold worker, from Guyana, sent me the following note:
Dr. Ron,
My colleagues use a “wet” gold technique to measure gold alloy density. Is this valid? Where does the formula come from?
Sincerely,
Ron
Well, to tell the truth, I had never heard of it and was skeptical. How can you measure density (mass/volume) by only measuring weight? So, I investigated. The technique claims that one can measure density with only a scale, by measuring the alloy’s weight in air and in water.
I could find no derivation, so I thought about it and derived it on my own. As far as measurements go, as stated, you only have to measure the weight in air and water. If you don’t have a scale that can handle being immersed in water, you can use a hanging scale (think weighing a fish). So, after weighing the alloy in air, you immerse it in water. It will weigh the amount of water it displaces less. The derivation is below:
As an example, let’s say you have a gold alloy ingot that weighs 1,000 grams (OK, I know grams is mass, but we are all sloppy and use it as weight, too) in air. You weigh it in water and it weighs 930 grams. From the formula below, the alloys density is:
r = 1000/(1000-930) = 14.29g/cc
Since the density of gold is 19.3g/cc, the alloy is not pure gold. If you knew the alloying element, say copper, you could use Indium’s Solder Alloy Density Calculator to determine that the alloy was 69.8% gold, 30.2% copper. If there are multiple alloying elements, since most of the common elements have a density of about 9 g/cc, you can even estimate the fineness of the gold.
Could this technique be used to measure the alloy density of say a handful of solder preforms. Sure, you could put them in a woven bag of non-hygroscopic material and weigh them in air and water. Admittedly, measuring the density of solder paste, with this technique, would be a challenge.
Cheers,
Dr. Ron