Researchers have released new data further explaining the limitations of indium addition in LEDs. It looks like this is finally an explanation as to why we only see indium being used in certain LED applications.
 I discussed this with Indium Corporation Research Technologist David Socha, who was familiar with the news release and always knows how to break down chemistry for non-chemists like myself.
I discussed this with Indium Corporation Research Technologist David Socha, who was familiar with the news release and always knows how to break down chemistry for non-chemists like myself.
Jim: “In general, how would you sum up this news [mentioned above]?”
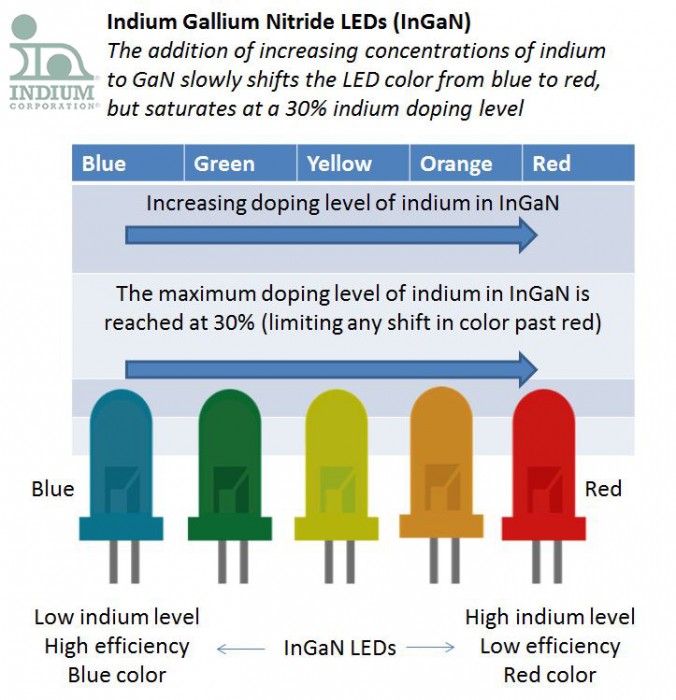
David: “This was an interesting article explaining why InGaN LEDs cannot be doped with greater than 30% indium.”
Jim: “Indium is used to create some of the different colored LEDs, how does that work?”
David: “As the indium concentration in InGaN increases, indium shifts from the +3 oxidation state to what scientists are calling "elastically frustrated rehybridization". This saturates the indium atoms with 4 nitrogen atoms (at 25-30% indium doping). This also shifts the LED color from blue through red as the indium concentration increases.”
Jim: “How does this relate to the LED colors that indium is not used for?”
David: “This explains why is it hard to tune the LED color past green when using an InGaN material system.”
Jim:“Okay, but manufacturers already knew that they couldn’t use InGaN to produce certain LEDs, right?”
David: “Scientists always knew that there was a fundamental limit when doping GaN with indium to shift the color towards red. It is only recently that they can now explain why this is the case.”
I’m thankful that David helps me stay up-to-date with the latest indium-related technology!
~Jim

