In previous posts, we have mentioned soldering to gold, but never discussed the unique processing aspects related to this.
Soldering to Gold
Soldering to copper is straight-forward.It is done all the time and the typical solders (SnPb or Pb-free) work extremely well on this surface.The only special requirements for this are that the copper is clean and not overly oxidized.Soldering to gold is done nearly as often, but is not as simple.
There are a number of factors related to surface finish which affect solder joint quality on gold.
Soldering to gold rarely implies using a tin solder reflowed directly to gold and forming an intermetallic with gold alone.If that is the case, we must be concerned over the strength of this joint because tin scavenges gold at a high rate, leaving a large proportion of voids at the interface, and the intermetallic formed is very brittle.
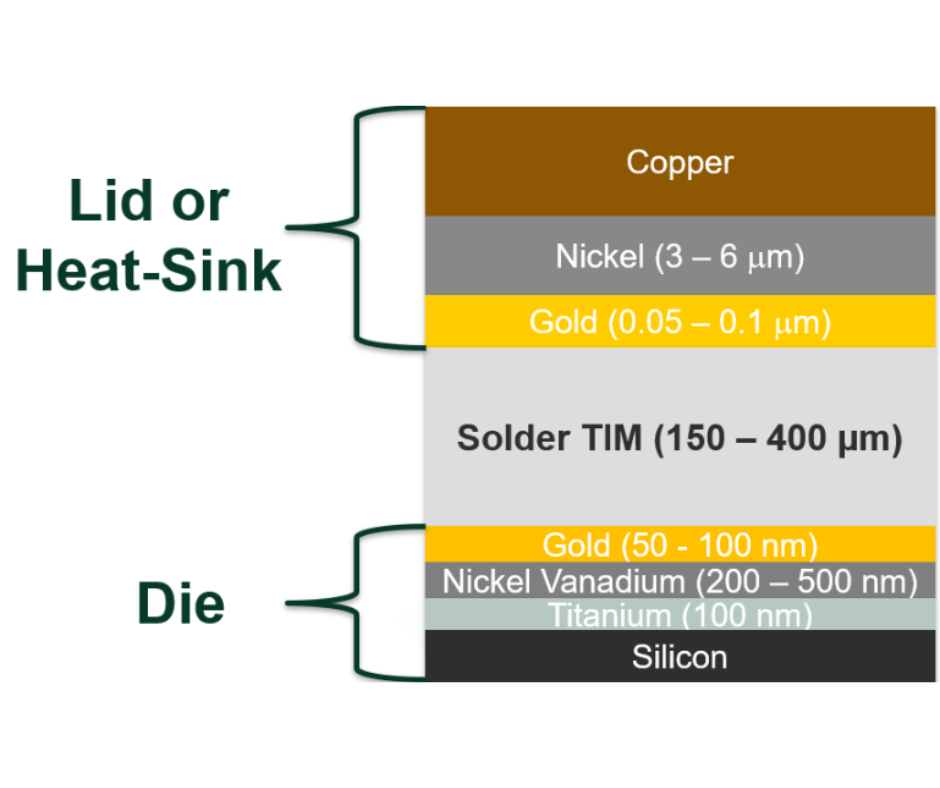
Typically, soldering to gold actually involves thin gold plating over nickel and the entire gold thickness is consumed into the solder joint.The resulting solder bond is between the nickel and the solder alloy.The function of the gold is as an oxidation barrier over the underlying metal (nickel in this case).This surface finish is often referred to as ENIG.
An appropriate gold thickness is between 10 and 20 micro-inches.This thickness is great enough to fully cover the underlying nickel, while thin enough to be fully consumed into the solder joint.When this gold is consumed, it makes the solder look grainy, but the joint will be more robust than if all the gold was not consumed.
For the solder to bond with a standard flux, the nickel under the gold plating must be clean.Most precious-metal platers are aware of this; however, care must be taken to clean the nickel prior to gold plating in any soldering locations.
Click here for more information on soldering to gold.